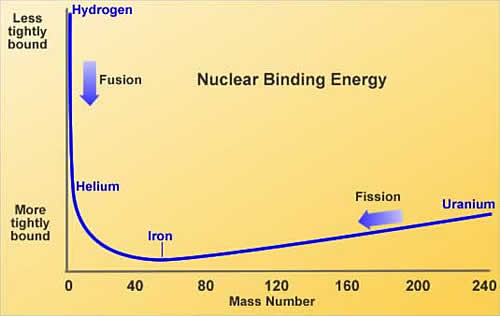
Fusion vs Fission
Source: NASA Goddard Space Flight Center, CC BY 2.0, via Wikimedia Commons
In fission, energy is gained by splitting heavy atoms, for example uranium, into smaller atoms such as iodine, caesium, strontium, xenon and barium, to name just a few. However, fusion is combining light atoms, for example two hydrogen isotopes, deuterium and tritium, to form the heavier helium. Both reactions release energy which, in a power plant, would be used to boil water to drive a steam generator, thus producing electricity.
Fission and chain reactions
Fission is the nuclear process that is currently run in nuclear power plants. It is triggered by uranium absorbing a neutron, which renders the nucleus unstable. The result of the instability is the nucleus breaking up, in any one of many different ways, and producing more neutrons, which in turn hit more uranium atoms and make them unstable and so on. This chain reaction is the key to fission reactions, but it can lead to a runaway process resulting in nuclear accidents. In conventional nuclear power stations today, there are systems in place to moderate the chain reactions to prevent accident scenarios and stringent security measures to deal with proliferation issues.
Fusion: inherently safe but challenging
Unlike nuclear fission, the nuclear fusion reaction in a tokamak is an inherently safe reaction. The reasons that have made fusion so difficult to achieve to date are the same ones that make it safe: it is a finely balanced reaction which is very sensitive to the conditions – the reaction will die if the plasma is too cold or too hot, or if there is too much fuel or not enough, or too many contaminants, or if the magnetic fields are not set up just right to control the turbulence of the hot plasma. This is why fusion is still in the research and development phase – and fission is already making electricity.
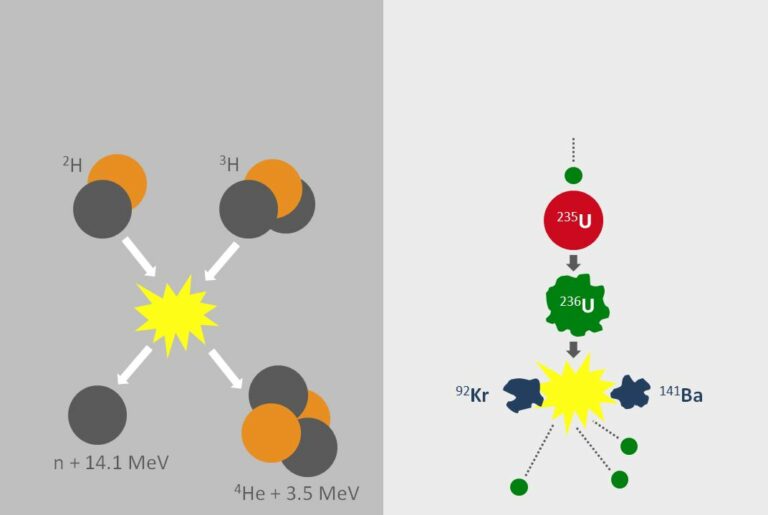
Binding energy
The key to why some atoms split and release energy while others fuse to do the same lies in how tightly the protons and neutrons are held together. If a nuclear reaction produces nuclei that are more tightly bound than the originals then energy will be produced by fusion, and for fission the opposite is true.
It turns out that the most tightly bound atomic nuclei are around the size of iron, which has 26 protons in the nucleus. So, one can release energy either by splitting very large nuclei, like uranium with 92 protons, to get smaller products, or fusing very light nuclei, like hydrogen, with just one proton to get bigger products.